EVICR.net – European Vision Institute Clinical Research Network is a network of Ophthalmological Clinical Research Sites, dedicated to perform multinational clinical research in ophthalmology with the highest standards of quality, following the European and International Directives for Clinical Research according to harmonised SOPs in order to strengthen the capacity of the European Union to study the determinants of ophthalmic diseases and to develop and optimise the use of diagnostic, prevention and treatment strategies in ophthalmology.
EVICR.net is an independent European Economic Interest Grouping (EEIG) established in 2010 in accordance with the Council Regulation (EEC) n.º 2137/85.
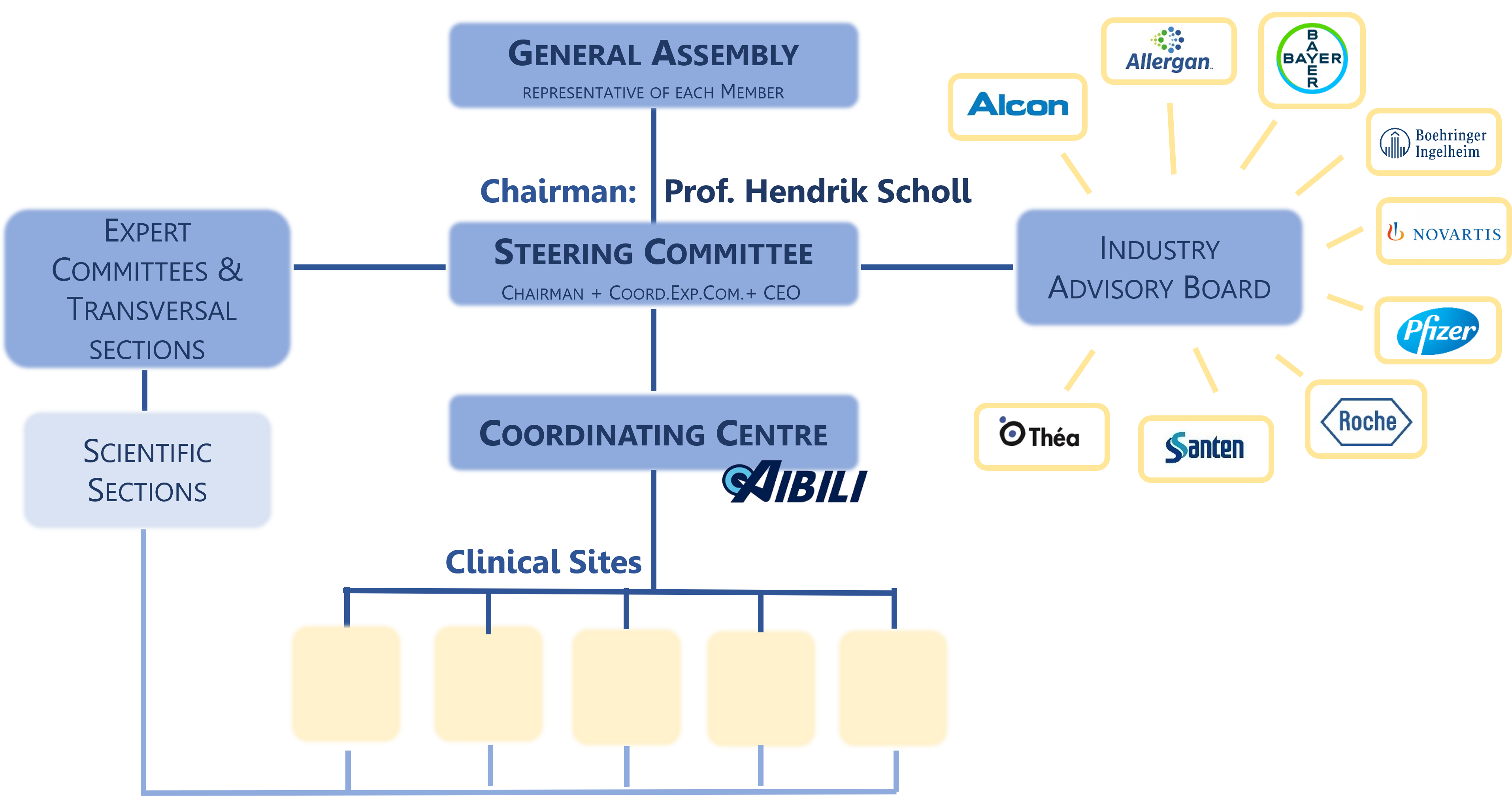
The EVICR.net is composed by the following bodies:
1. The General Assembly consists of all EVICR.net Members, at present, 95 Clinical Sites from 16 Countries. The General Assembly is the supreme organ of the Network and is responsible for the governing statutes to regulate the day-to-day business.
2. The Steering Committee is responsible for the activities of the EVICR.net and acts as its decision-making body within the framework set by the General Assembly. The Steering Committee consists of up to eleven representatives: the Chairman, the Coordinator of each Expert Committee, the Coordinator of each Transversal Section and the CEO of the Management Board.
The present Steering Committee is constituted by the following members:
- Hendrik Scholl, MD, MA, the EVICR.net Chairman and Retinal Dystrophies Expert Committee Coordinator
- Francesco Bandello, MD, PhD, Age-Related Macular Degeneration Expert Committee Coordinator
- José Cunha-Vaz, MD, PhD, Diabetic Retinopathy & Vascular Diseases Expert Committee Coordinator
- Francesca Cordeiro, MD, PhD, Glaucoma Expert Committee Coordinator
- Marie-José Tassignon, MD, PhD, Anterior Segment Expert Committee Coordinator
- Pasquale Aragona, MD, PhD, Ocular Surface, Inflammation, Dry-Eye & Allergies Expert Committee Coordinator
- Birgit Lorenz, MD, PhD, Rare Diseases Transversal Section Coordinator
- Tunde Peto, MD, PhD, FHCO, FHCD, Reading Centres Transversal Section Coordinator
- Cecília Martinho, BSc, Coordinating Centre / Management Board CEO
3. The Coordinating Centre / Management Board is located at AIBILI, in Coimbra, Portugal. The Management Board functions, within the framework set by the Steering Committee, are general network management activities, management of members and management of activities directly related to clinical studies.
4. The Expert Committees have a fundamental role in the scientific organization of EVICR.net and cover the following main areas of research:
-
- Age-Related Macular Degeneration – Coordinator: Francesco Bandello
- Retinal Dystrophies – Coordinator: Hendrik Scholl
- Diabetic Retinopathy & Vascular Diseases – Coordinator: José Cunha-Vaz
- Glaucoma – Coordinator: Francesca Cordeiro
- Anterior Segment – Coordinator: Marie-José Tassignon
- Ocular Surface, Inflammation, Dry-Eye & Allergies – Coordinator: Pasquale Aragona
There are also the following Transversal Sections that work together with the Expert Committees, when applicable:
-
- Rare Diseases – Coordinator: Birgit Lorenz
- Reading Centres – Coordinator: Tunde Peto
The Expert Committee and the Transversal Sections will advise the Steering Committee on matters related to the running of clinical research in their scientific area.
The Expert Committees functions are to provide expert advice for Industry; evaluate IIR submitted by members; propose clinical studies to Industry or for European Union funding; evaluate EVICR.net potential for a specific trial and establish Working Groups whenever necessary.
5. Each Expert Committee supervises a Scientific Section with the participation of the subspecialty representatives from each EVICR.net Clinical Site Member. Each Clinical Site will then nominate one representative for each area of interest and expertise. This representative will be part of the chosen Scientific Section.
6. The Industry Advisory Board advises the Steering Committee in all matters of strategic relevance, particularly pertaining collaborations with Industry. The Industry Advisory Board is composed of individuals or representatives of entities who have given support for the activities of EVICR.net, by invitation of the Steering Committee.
The Industry Advisory Board Members are: Alcon, Allergan, Bayer, Boehringer Ingelheim, Pfizer, Novartis, Santen, Théa and Roche.